The Science Behind Dry Ice and Liquid Nitrogen Ice Cream
Two cooling agents used pretty uniquely in molecular gastronomy are dry ice and liquid nitrogen, which are molecules that are commonly found in nature but are in a different form.
Dry ice is solid carbon dioxide, the gas version of which is in the air (at about a .035% concentration), in our bodies as we produce energy from glucose, and in carbonated drinks. The reason we typically only see carbon dioxide as a gas or as a solid has to do with its phase diagram:
The state of a substance (i.e. whether it’s a solid, liquid or gas) is dependent on two factors: pressure and temperature. Pressure on Earth is typically around 1 atmosphere, while temperature can vary depending on the location. As can be seen in the diagram, at 1 atm, carbon dioxide can either be a solid or a gas, but not a liquid; only at a pressure of 5.11 atm can we begin to see carbon dioxide in a liquid form.
Unlike water, which freezes at zero degrees Celsius, carbon dioxide freezes at -78.5 degrees Celsius. This is because of the great difference in their intermolecular forces. Water molecules experience hydrogen bonds with other molecules because they have such a strong dipole moment, meaning that the oxygen atom has such a strong pull on the hydrogen atoms’ electrons that the molecule develops a positive and negative charge. These charges are attracted to the opposite charges of other molecules, forming a tight-knit group of molecules, so water is a substance that is easy to freeze.While oxygen atoms have a great pull on carbon’s electrons in the carbon dioxide molecule, the symmetrical shape of the molecule gives a neutral charge on both sides. Because of this, carbon dioxide has few intermolecular forces and it is more difficult to force them to stick together as a solid as opposed to a free-flowing gas.
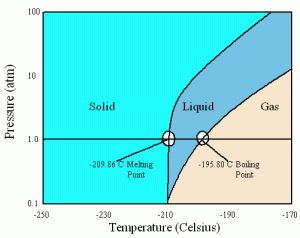
Liquid nitrogen, on the other hand, is usually seen in either liquid or gas form. Nitrogen, or N2, has a completely symmetrical structure with an extremely strong triple bond between the two nitrogen atoms. It has virtually no intermolecular forces, especially because the two nitrogen atoms attract electrons equally well, so it is extremely hard to force nitrogen molecules into liquid or solid states. Unlike carbon dioxide, nitrogen can exist in all three states at 1 atm:
Its boiling point, at -195.8 degrees Celsius, is also lower than carbon dioxide’s freezing point, making liquid nitrogen colder than solid carbon dioxide.
Now, the question left is: why does pouring/adding dry ice or liquid nitrogen to a substance freeze it? The answer may seem simple (well, it’s cold!), but it’s a little more complicated than that. Temperature, be it in Fahrenheit, Celsius, or Kelvin, is a measure of energy (of the kinetic variety). When substances are surrounded by temperatures above their freezing/boiling points, the energy from that higher temperature will flow into the substance with the lower temperature in order to begin melting or subliming that substance. Until all of the substance is melted or sublimed, the unchanged portion of that substance will maintain the same temperature. It’s kind of confusing, so here’s an example: liquid nitrogen’s boiling point is at -195.8 degrees Celsius. If we pour one liter of it into a sorbet base at room temperature (about 25 degrees Celsius), the energy from the sorbet will go into evaporating the liquid nitrogen, thereby cooling the sorbet base and reducing the amount of liquid nitrogen. As long as there is liquid nitrogen in the bowl, though, its temperature will stay at -195.8 degrees. Eventually, when all of the liquid nitrogen has evaporated, the sorbet is probably at a temperature below the freezing point for water because so much energy has been removed from it. As such, at that point it would be a solid sorbet. It can be demonstrated by this graph:

Substances do not change temperature when they are in the process of changing phases. That’s the basic principle behind dry ice and liquid nitrogen ice creams.
Leave a comment