Recently, I tried making effervescent caramels, which were not super successful. However, the chemistry behind the particular type of effervescence I created is pretty simple and easy to understand.
The two additives I combined for the effervescent coating were citric acid and sodium bicarbonate. Citric acid is commonly used for both flavoring citrusy foods and acidifying solutions because it is found in many citrus fruits. It is also very important physiologically; it is involved in the oxidation of fats and proteins and it also facilitates the conversion of carbohydrates into carbon dioxide and water during cellular respiration. (Cellular respiration is the process by which we convert food into energy.) At room temperature, it exists as a white powder, which makes it easy to use in molecular gastronomy.

Don’t get any funny ideas.
Sodium bicarbonate is the main ingredient in baking soda and is a very unique chemical compound. It is amphoteric, which means that it can react with both acids and bases to neutralize the pH of both. Because of this, though, it is neither a very strong base nor a very strong acid, meaning that it can really only neutralize weak acids and bases.
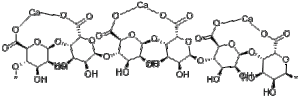
Luckily for this reaction, citric acid is a weak acid! The chemical reaction that takes place between sodium bicarbonate and citric acid is as follows:

As can be seen from the fabulous diagram, the rightmost hydroxide functional group (OH) on the sodium bicarbonate combines with the leftmost carboxylic acid (COOH) on the citric acid to form carbonic acid. The leftover parts bond to make sodium citrate. Carbonic acid is a fairly weak compound and will fall apart, yielding water and carbon dioxide. This carbon dioxide is what we feel as the fizzy part of the reaction.
So why are we able to coat caramel with this mixture of powders without the fizz escaping right away? The compounds need an aqueous environment (more plainly stated, they need to be dissolved in water) to be able to interact enough to react. When the caramels are ingested, the saliva in your mouth provide enough moisture to allow this reaction to occur. As long as they are dry, the reagents won’t undergo the acid-base reaction to form fizzy carbon dioxide.
Well, that was probably the simplest chemical reaction that I’ve researched so far. Effervescence is extremely easy to create, even for novices in molecular gastronomy. I’m going to continue reviewing molecular gastronomy as I design my lesson plans, so stay tuned for more posts.