The Science Behind Spherification
Earlier this week, I did spherification for the first time; you can find the link here. I searched into why this process occurs, and I found some interesting results.
The two main components involved in spherification are alginate strands, usually found in the additive sodium alginate, and calcium ions, which can come from calcium chloride, calcium lactate, or calcium gluconate. One of these ingredients is dissolved into a distilled water bath, while the other is dissolved in the liquid you want to spherify. It depends on whether you want to do basic spherification or reverse spherification based on the liquid’s properties.
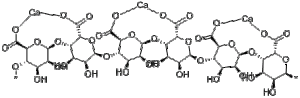
The structure of an alginate strand is as follows:

When sodium ions (Na+) are bonded to the oxygens, the strand is fairly flexible and soluble in liquid. If a solution containing sodium alginate is dropped into a calcium ion bath, however, the calcium ions bond to the alginate strands, replacing the sodium ions. Calcium ions have a positive two charge (Ca2+), and therefore must make two bonds to complete their electron shell and become stable molecules. Because they are taking the place of sodium ions, they must make an additional bond to satisfy this requirement. The result looks something like this:
The molecules are all stuck together in a huge network! This eliminates their flexibility, making them more rigidly bonded together. To us, this looks and feels like a thin gel, which makes up the spheres’ thin membrane.
There are a few caveats that we have to keep in mind when spherifying liquids. First, if a liquid contains a non-negligible amount of calcium, we must do reverse spherification as opposed to basic spherification (reverse spherification is the process of mixing calcium ions into the liquid and making a sodium alginate bath instead of the other way around). Doing basic spherification with this type of liquid would cause the alginate molecules to prematurely react with the calcium in the liquid, forming a huge network of alginate strands within the whole body of the liquid, instead of just making a small membrane around it. Reverse spherification solves this problem by increasing the calcium concentration in the liquid so that when dropped in the alginate bath, only the outer calcium ions react with the strands of alginate, forming a sphere.
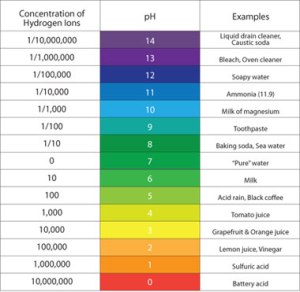
The second problem is when the liquid’s pH is too low. This may not seem like a significant problem at first, but can pose a real challenge to spherifying citrusy liquids. A low pH indicates a high concentration of hydrogen ions (H+), as shown by this chart:
When pH levels are below 5, the concentration of hydrogen ions is very large, and this increases their reactivity. When alginate molecules are in contact with a liquid with both calcium ions and a low pH, many of the molecules will react with the hydrogen ions instead of the calcium ions. Hydrogen ions have the same charge as sodium ions, so they will not need to bond with other alginate strands to complete their electron shells. As a result, the alginate strands will remain as flexible as they were with the sodium ions and no gel will form. We must add sodium citrate to liquids with low pHs to react with the excess hydrogen ions, allowing the calcium ions to react with the alginate strands more readily.
Finally, basic spherification does not last a long time because of one simple reason: there are too many alginate strands within the sphere to go unreacted for an extended period of time. The calcium ions will find a way to react with the remaining alginate molecules, and as they do so, the huge network of atoms that makes up the membrane will extend inwards. The liquid inside the membrane will eventually all be part of the network and it will feel more like a squishy gel than caviar. This does not happen with reverse spherification because the calcium ions within the membrane have nothing to react with; the alginate strands have either already all reacted or been rinsed of after spherification. This leaves the liquid on the inside with its original lack of a rigid structure.
Well, that was a long explanation of spherification. Even though it may sound complicated, it really is one of the most simple reactions in molecular gastronomy because it has so few reactants and products. Next time, I’ll be trying gelification. It’ll be fun!
June 20, 2013 at 9:12 AM
So if i was to describe this in simple terms would you say that in reverse spherification the sodium glucomate sucks the calcium to the outside forming the gel membrane and in a way, trying to ‘dehydrate’, as sodium, salt, is known for dehydration. Trying to think of an easy way of describing this to some students.
Many thanks
June 20, 2013 at 4:44 PM
The way I described it to my (5th-8th grade) students was that the calcium in one liquid replaced the sodium in the other, forming a jumbled up web of atoms around the edge of the drop. The web is so clustered and messy, no liquid is allowed to pass through it, so the inside stays liquid, like caviar. I’m not sure any dehydration occurs here, but if your students better understand it this way, I commend you for thinking of it. 😀
Thanks for reading!
May 12, 2014 at 7:14 PM
Reverse spherification is sort of like adding chromium to steel. The chromium reacts with oxygen to form a protective layer that prevents the iron from reacting and rusting just as the calcium reacts with the excess alginate to form a protectiver layer around the liquid preventing the rest from reacting.